Sulfuric acid, a highly corrosive and dense liquid, is one of the most widely produced and consumed chemicals globally. Its applications span across various industries, including fertilizers, pharmaceuticals, and petroleum refining. With a chemical formula of H2SO4, sulfuric acid is known for its strong dehydrating properties and its ability to react with most metals and organic materials. The production of sulfuric acid involves the contact process, which includes the oxidation of sulfur dioxide to sulfur trioxide, followed by the reaction of sulfur trioxide with water to form sulfuric acid.
Key Points
- Sulfuric acid is a key component in the production of fertilizers, accounting for approximately 60% of its global use.
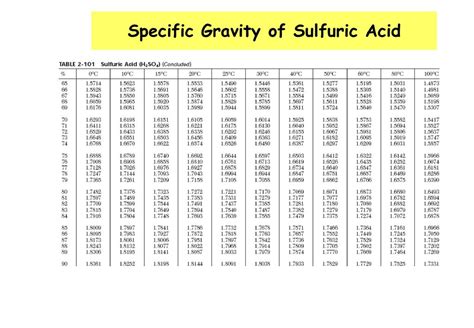
- The acid has a high density of 1.83 g/cm³, making it one of the densest liquids among common chemicals.
- Sulfuric acid is highly corrosive and can cause severe burns upon contact with skin, necessitating careful handling and storage.
- It plays a crucial role in the petroleum industry, primarily used in the alkylation process to produce high-octane gasoline.
- The annual global production of sulfuric acid exceeds 250 million tons, underscoring its importance in various industrial processes.
Chemical Properties and Uses
Sulfuric acid’s chemical properties make it an indispensable chemical in various industrial applications. Its ability to donate a proton (H+ ion) makes it a strong acid, capable of reacting with bases to form salts and water. This property is crucial in the production of fertilizers, where sulfuric acid is used to produce phosphoric acid, a key ingredient in the manufacture of ammonium phosphate fertilizers. Additionally, sulfuric acid is used in the pharmaceutical industry for the synthesis of various drugs and in the production of dyes, pigments, and explosives.
Production Process
The production of sulfuric acid involves several steps, starting with the extraction of sulfur, which is then burned to produce sulfur dioxide (SO2). The sulfur dioxide is then oxidized to sulfur trioxide (SO3) using a vanadium pentoxide catalyst in the contact process. Finally, the sulfur trioxide is reacted with water to produce sulfuric acid. This process requires careful control of temperature and pressure to optimize yield and minimize the formation of by-products.
| Chemical Reaction | Equation |
|---|---|
| Sulfur combustion | S + O2 → SO2 |
| Oxidation of SO2 | 2SO2 + O2 → 2SO3 |
| Formation of H2SO4 | SO3 + H2O → H2SO4 |

Environmental and Health Concerns

The production and use of sulfuric acid pose significant environmental and health risks. The release of sulfur dioxide into the atmosphere contributes to acid rain and air pollution, affecting both human health and the environment. Direct contact with sulfuric acid can cause severe burns and eye damage, emphasizing the need for stringent safety measures in handling and storage. Efforts to mitigate these risks include the implementation of stricter emissions controls and the development of safer handling practices.
Safety Measures and Regulations
Given the hazardous nature of sulfuric acid, regulatory bodies have established strict guidelines for its handling, storage, and disposal. These regulations aim to minimize exposure risks and prevent environmental contamination. Industries using sulfuric acid are required to implement safety protocols, including the use of personal protective equipment, ventilation systems, and spill containment measures. Moreover, there is a growing emphasis on developing more sustainable and less hazardous alternatives for certain applications.
What are the primary uses of sulfuric acid in industry?
+Sulfuric acid is primarily used in the production of fertilizers, in the petroleum industry for refining, and in the manufacture of chemicals such as phosphoric acid, nitric acid, and hydrochloric acid.
What safety precautions should be taken when handling sulfuric acid?
+When handling sulfuric acid, it is essential to wear protective clothing, including gloves, safety glasses, and a face shield. Additionally, operations should be conducted in well-ventilated areas, and spill response plans should be in place.
How does sulfuric acid contribute to environmental pollution?
+Sulfuric acid production leads to the release of sulfur dioxide, a precursor to acid rain and a contributor to air pollution. Moreover, improper disposal of sulfuric acid can lead to water and soil contamination.
In conclusion, sulfuric acid is a versatile and widely used chemical with applications across multiple industries. Its unique properties make it an essential component in various processes, from fertilizer production to petroleum refining. However, its handling and production also pose significant environmental and health risks, emphasizing the need for strict safety protocols and regulatory measures to mitigate these impacts.